In a recently published experiment [1] at CERNs nuclear physics facility ISOLDE, the electron affinity of astatine has been determined by a group of international scientists.
Astatine is the rarest naturally occurring element on earth, with an estimated total presence of only 70 mg in earths crust. It is a purely radioactive element, with its longest living isotope, 210At having a lifetime of only 8.1 h. Apart from the fundamental interest in determining the EA of the heaviest halogen, there is also a practical application for astatine: its isotope 211At is a promising candidate for targeted alpha therapy, a cancer treatment using alpha emitters to selectively kill cancer cells. However, due to the scarcity of astatine, its chemical properties, needed to develop radiopharmaceuticals using astatine, remained illusive. Hence, in order to benchmark chemical models, experimental determination of fundamental atomic properties such as the ionization potential, previously measured at ISOLDE [5], and the electron affinity are of great importance.
The electron affinity (EA) is the energy gained when an additional electron is attached to a neutral atom, creating a negative ion. It is one of the fundamental properties of an element, giving insight into its chemical behaviour. When combined with the ionization potential, chemical properties like the electronegativity can be derived, revealing the character of bonds astatine forms with other elements.
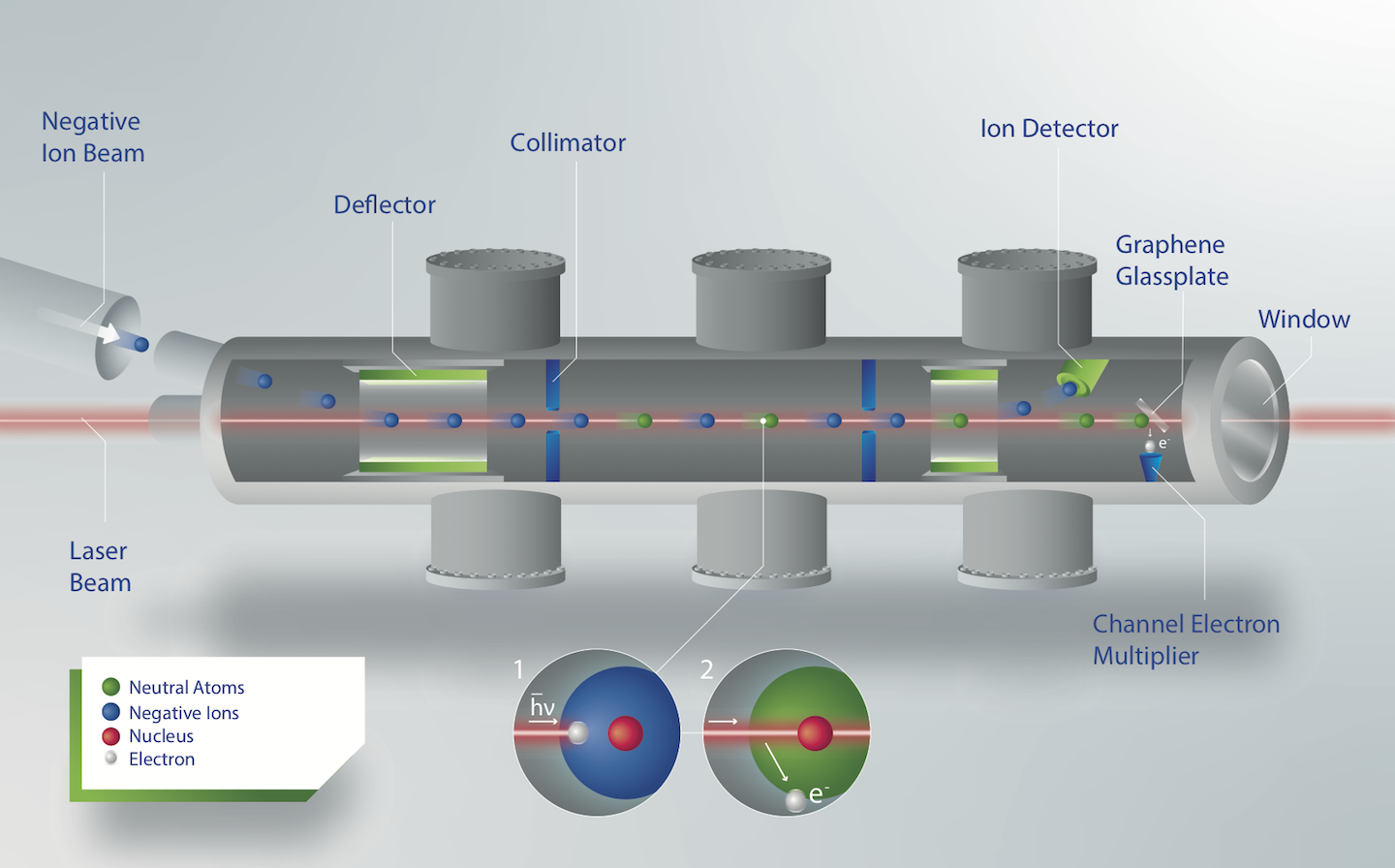
In order to produce astatine at ISOLDEs General Purpose Separator (GPS) beamline, the 1.4 GeV proton beam from the Proton Synchrotron Booster (PSB) impinged upon a thorium target, creating astatine isotopes in a spallation re- action. The reaction products diffused through a tantalum transfer line into an ion source, both held at a temperature of around 1500 C, where they were ionized negatively by a LaB6 surface ionizer. Subsequently, the ions were extracted with a beam energy of 20 keV and mass separated with the GPS separator magnet. The resulting negative ion beam of 211At ions was sent to the Gothenburg ANion Detector for Affinity measurements by Laser PHotodetachment (GANDALPH), shown in Fig. 1.
Here, the ion beam was overlapped parallel with a frequency tuneable laser beam, resulting in the neutralization (photodetachment) of ions, as shown in inlets of Fig. 1. While residual charged particles where bend into a Faraday cup, neutralized atoms impinged on a graphene coated quartzplate, creating secondary electrons, which were subsequently detected using a channel electron multiplier. By measuring the neutral particle yield as a function of the laser frequency, the photodetachment threshold representing the EA was extracted, as shown in Fig. 2. The experimental data is fitted to the Wigner law which describes the photodetachment cross section around the threshold, while addi- tionally taking the hyperfine splitting of the ground state of the neutral 211At atom (determined by Cubiss et al. at ISOLDE [6]) into account. This yielded an electron affinity of 2.415 78(7) eV for astatine. With this result, a 10-year measurement campaign to determine the fundamental properties of astatine was successfully completed, facilitating the further development of astatine as radiopharmceutical as well as future EA measure- ments of heavier elements.
References:
[1] David Leimbach et al. The electron affinity of astatine. Nature Communi- cations, 11(1):1–9, July 2020.
[2] Sebastian Rothe et al. Laser photodetachment of radioactive 128I. Journal of Physics G: Nuclear and Particle Physics, 44(10):104003, 2017.
[3] D. Leimbach et al. Upgrades of the gandalph photodetachment detector towards the determination of the electron affinity of astatine. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 463:277 – 279, 2020.
[4] J. Warbinek et al. A graphene-based neutral particle detector. Applied Physics Letters, 114(6):061902, 2019.
[5] S. Rothe et al. Measurement of the first ionization potential of astatine by laser ionization spectroscopy. Nature Communications, 4:1835, may 2013.
[6] J. Cubiss et al. Charge radii and electromagnetic moments of 195−211At. Physical Review C, 97:054327, May 2018.
